C. Light Selected for Roche MS Innovation Challenge; Multi-Site Trial to Study Eye Movements as Biomarker of Disease Progression
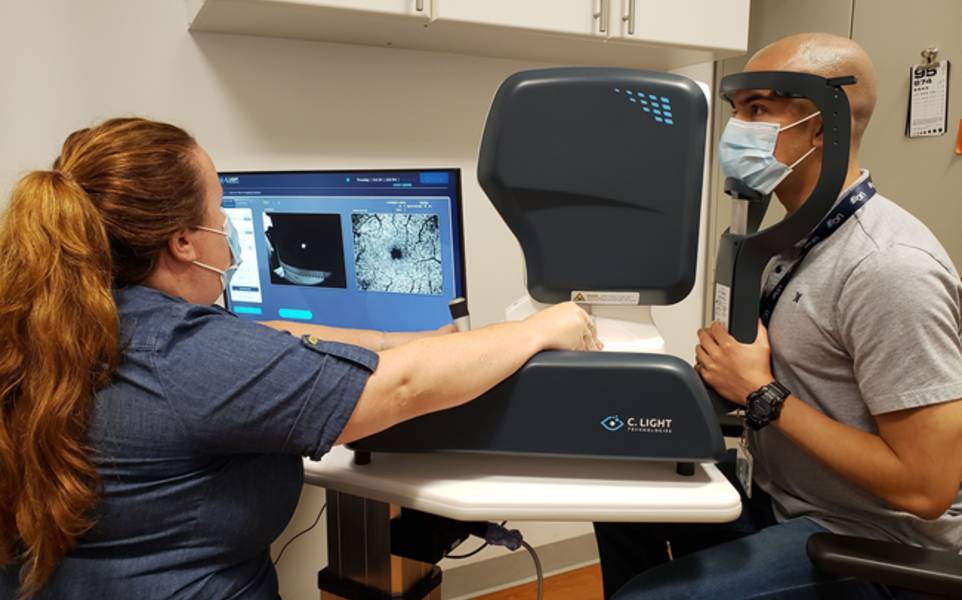
UCSF and Sutter Health to participate in two-year study using retinal eye-movement monitoring
C. Light Technologies has been named one of five projects selected for funding through the Roche MS Innovation Challenge. The support will enable a two-year clinical study with multiple sclerosis (MS) centers at the University of California, San Francisco (UCSF) and Sutter Health to evaluate whether microscopic fixational eye movements can serve as a non-invasive biomarker of MS progression.
The trial will use C. Light’s Retitrack scanning laser ophthalmoscopy (SLO) device, an FDA 510(k)-cleared retinal eye-movement monitor. Participants will undergo eye-movement recording sessions at five intervals over the course of two years. Researchers aim to determine whether changes detected by Retitrack can predict progression independent of relapse activity (PIRA) earlier than existing measures, including the Expanded Disability Status Scale (EDSS) and MRI. The study will also include participants receiving B-cell depleting therapies.
Earlier work from C. Light indicates that fixational eye-movement patterns change with MS and correlate with EDSS progression. The Retitrack system measures involuntary microscopic eye movements during a 10-second scan without requiring dilation.
C. Light CEO Christy Sheehy-Bensinger said the research is intended to generate new, objective data that could help identify MS progression before ambulatory symptoms appear. Chief Medical Officer Jacqueline Theis said the technology may support earlier detection of PIRA, more precise evaluation of therapeutic effects, and improved patient monitoring.
In July, the American Medical Association created CPT code 1010T for retinal eye tracking, which becomes effective Jan. 1, 2026 and may enable insurance reimbursement for Retitrack scans.
The company recently closed a $3 million seed extension round to support an upcoming FDA clinical trial focused on adding AI-based disease detection capabilities to the Retitrack system. C. Light has also joined the DayOne Accelerator program in Basel, Switzerland, and will participate in its Innovation Showcase on Dec. 10–11 at the Roche campus.






