In its 11th annual Healthcare Fraud & Abuse Review, Bass, Berry & Sims provides analysis of healthcare fraud enforcement actions and False Claims Act (FCA) developments probing COVID-19 relief, telehealth, opioid enforcement and other areas.
In 2022, the federal government set the stage for wider scrutiny of billions of dollars in pandemic relief funds for healthcare provider groups in 2023 and beyond, as described in the Healthcare Fraud & Abuse Review 2022 published by Bass, Berry & Sims.
In the 11th annual edition of the Review, Bass, Berry & Sims expects more enforcement actions related to funds distributed under the Coronavirus Aid, Relief, and Economic Security (CARES) Act in 2023 and other federal relief programs.
Download the Healthcare Fraud & Abuse Review 2022.
“Last year, DOJ made clear its intention to pursue fraud cases related to provider relief funds, from appointing a Director for COVID-19 Fraud Enforcement to setting up Strike Force Teams dedicated to these cases,” said Brian D. Roark, co-chair of the firm’s Healthcare Fraud & Abuse Task Force. “We expect that this is the beginning of a years-long effort to pursue cases concerning provider relief funds that may involve far more complex circumstances than the blatant fraud that DOJ has prosecuted so far. We also expect whistleblowers to target alleged fraud in use of COVID-19 relief funds in qui tam cases filed under the False Claims Act.”
Bass, Berry & Sims will host a complimentary webinar that will provide an overview and discussion of key focus areas covered in the Review on Wednesday, February 22, from 10:00 a.m.-12:00 p.m. CT. Please click here to register.
Government More Proactive
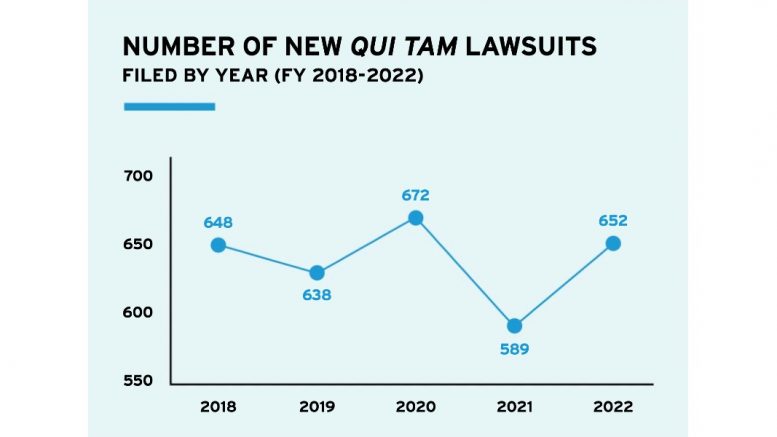
The Department of Justice’s (DOJ) proactive focus on fraud in COVID-19 relief programs fits a pattern of more government-initiated healthcare fraud enforcement activity overall. In each of the past two years, DOJ has initiated more FCA cases than in any single year since 1995. In the fiscal year that ended Sept. 30, 2022, DOJ recovered $1.7 billion in FCA settlements and judgments related to federal losses involving the healthcare industry. In keeping with percentages from prior years, healthcare-related recoveries represented the overwhelming majority of the $2.2 billion in total FCA settlements in fiscal year 2022, nearing 80%. The total number of FCA settlements, at 351, was the second-highest number in a single year, although recoveries were down from $5.6 billion in fiscal year 2021.
“The government has continued to invest in its own data analytics and data mining, which has greatly enhanced DOJ’s ability to pursue healthcare fraud cases,” said Matthew M. Curley, co-chair of the Healthcare Fraud & Abuse Task Force and editor of the Review. “
Both COVID-19 relief funds and telehealth also remain the subjects of dedicated enforcement initiatives. With these moves, the federal government continues its proactive approach to healthcare fraud enforcement, in addition to its more traditional approach of investigating the allegations in qui tam cases brought by whistleblowers under the False Claims Act.”
In July 2022, DOJ announced criminal charges against 36 defendants in 13 federal districts alleging $1.2 billion in intended losses associated with fraud schemes involving telemedicine, cardiovascular and cancer genetic testing, and durable medical equipment (DME). At the same time, HHS-OIG issued a Special Fraud Alert concerning telehealth arrangements. The Special Fraud Alert focused on areas where healthcare providers and companies need to be cautious in telehealth arrangements, such as arrangements with limited patient-practitioner relationships and the compensation arrangements of practitioners.
Last year also brought expansion of the federal government’s criminal and civil enforcement related to the opioid epidemic. The success of DOJ’s Appalachian Regional Prescription Opioid (ARPO) Strike Force—which announced charges against 14 persons, including 12 medical professionals in May 2022—led to the creation of the New England Prescription Opioid (NEPO) Strike Force the following month.
“The federal government is scrutinizing every link of the opioid distribution chain, from manufacturers and distributors to pharmacies and individual prescribers,” said Lisa S. Rivera, a member of the Healthcare Fraud & Abuse Task Force and chair of the Firm’s Compliance & Government Investigations Practice Group. “This wide range of possible enforcement targets demonstrates the federal government’s commitment to addressing the opioid epidemic through enforcement efforts, as well as other areas of the broad-ranging Controlled Substances Act. Companies and providers should take great care to abide by all statutory and regulatory requirements related to opioid products and compliance with the CSA.”
Analysis of Major FCA Case Law Trends
The Review provides analysis of major trends in FCA cases law including an in-depth review of developments related to the Stark self-referral law and the Anti-Kickback Statute (AKS).
“Government fraud enforcers and whistleblowers alike continue to scrutinize financial relationships between hospitals, health systems and other provider organizations,” said Anna M. Grizzle, a member of the firm’s Healthcare Fraud & Abuse Task Force who advises clients on enforcement and compliance-related issues. “Healthcare providers must build a robust, up-to-date compliance program and always remain alert to practices that may violate this complex area of healthcare law.”
One of last year’s most significant settlements involved AKS-related allegations against pharmaceutical manufacturer Biogen, which agreed to pay $900 million to resolve a whistleblower lawsuit alleging that the company had offered and paid kickbacks to physicians in the form of speaker honoraria, speaker training fees, consulting fees and meals to induce the physicians to prescribe Biogen’s multiple sclerosis drugs.
This settlement and other AKS-related enforcement actions are covered in detail in the Review. The Review also highlights issues to watch this year and provides a comprehensive review of settlements involving the healthcare industry, grouped by sector:
- Hospitals and Health Systems
- Long-Term Care
- Pharmaceutical and Medical Device Companies
- Pharmacy Services
- Lab and Diagnostic Service Providers
- Behavioral Health
- Managed Care and Health Plans
- Specialty Care and Other Provider Entities
Download the Review here; visit the Bass, Berry & Sims Healthcare Fraud & Abuse Resource Center, which features a database of healthcare fraud settlements since 2012; and follow the Inside the False Claims Act blog to stay up to date on FCA matters.