4D Path, creator of a patented computer-aided cancer diagnostic and precision oncology platform, has extended its existing partnership with the University of Leeds in the UK through 2027, the two organizations announced today. Through a prior three-year partnership, 4D Path has already successfully completed three breast cancer clinical studies at Leeds with striking results. Cases from over 1,000 patients from a real-life hospital setting were analyzed across multiple trials using a fully automated 4D workflow.
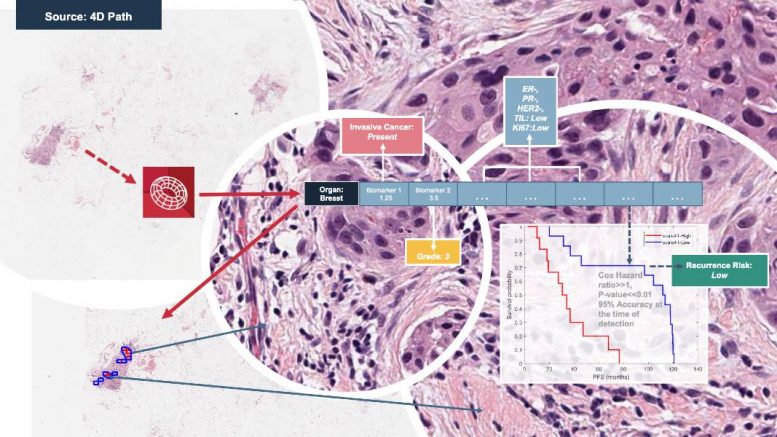
4D Path, which has raised $6.4 million in funding to date and has been awarded a coveted FDA Breakthrough Device Designation, applies a cloud-based, quantitative approach to find hidden data in biopsy and resection specimen pathology images. Its results to date hold the promise of significant improvements over the existing standard of care. Indeed, the initial histopathologist morphological diagnosis in the patient management pathway may no longer be the first step in a lengthy process towards biomarker profiling and stratification, but perhaps the only step, thus eliminating the need for additional expensive tests (e.g. immunohistochemistry, FISH, NGS).
“The anxiety and distress associated with waiting for a confirmed cancer diagnosis has a marked detrimental impact on patients and their families’ quality of life and finances,” said Dr. Debbie Beirne, NIHR Leeds CRF manager/deputy director and cancer patient and public involvement (PPI) group Lead, National Health Service (NHS). “Accelerating the diagnostic process without compromising accuracy would overcome this issue and offset the adverse effects that increasing workloads have on the quality of diagnostic assessments made by reduced numbers of overstretched histopathologists. Improving the speed of the diagnostic process supports the initiation of primary treatment at the earliest possible opportunity.”
The breast cancer investigations conducted so far at the University of Leeds using the 4D Q-plasia OncoReader Breast platform have focused on identifying the presence of breast cancer and determining its subtype, grade, HER2 and hormone receptor status from H&E stained whole slide biopsy images only. These involved 400+ patients and 700+ images scanned across two different leading scanner platforms to demonstrate the robustness of the device across image formats.
“We are very excited by our study results which, when validated in large scale follow-on studies, could revolutionize the way we perform diagnoses, interact with our oncology colleagues and support patients. This ongoing partnership capitalizes and builds on our internationally recognized expertise in digital pathology , says Dr. Rebecca Millican-Slater, NHS consultant breast histopathologist at Leeds Teaching Hospitals Trust (LTHT), who has been involved in the trial stated.
“One in eight women will be affected by breast cancer in their lifetime, and improving diagnostic accuracy at the level of biopsies is becoming increasingly important to support clinical practice aimed at minimizing the severity of surgical intervention,” said Mr. Rodrigo Navarro, CEO at 4D Path. “We’re grateful to the University of Leeds for giving us the opportunity to work in an academic clinical setting to demonstrate both our strength in delivering various diagnostic outcomes for breast cancer, and our ability to enable essential molecular tumor profiling.”
Going forward, 4D Path and the University of Leeds plan to expand the remit of their current trials across a range of other malignancies, with a specific focus on skin, lung, gynecological, and head and neck cancers. Professor Andrew Hanby, NHS consultant breast histopathologist at LTHT who has also participated in the studies, said: “We look forward to continuing our partnership with 4D Path in testing their OncoReader in the clinical setting. Their technology promises to significantly enhance the quality and efficacy of the diagnostic process, giving patients the assurance that their treatment pathway has a solid and safe foundation.”