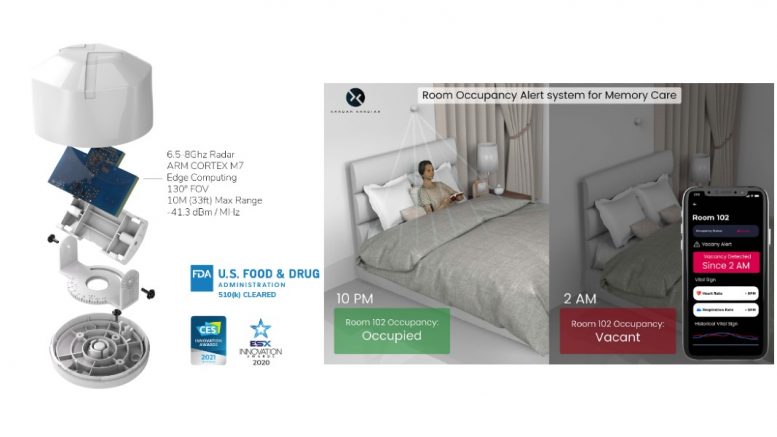
The world’s first and only commercially deployed radar people-counting solution enables continuous and completely autonomous vital sign readings in real-time
Xandar Kardian, the world’s first and only commercially deployed, non contact, continuous and completely autonomous health monitoring solution utilizing proprietary radar technology, announced today that its XK300 Autonomous Health Monitoring Solution has achieved 510(k) Clearance by the U.S. Food & Drug Administration, paving the way for a new era of accurate and reliable radar technology. Designed for general ward (hospital) and senior nursing homes (including memory care), Xandar Kardian provides critical early deterioration detection in both hospital and home settings by taking continuous measurements of resting heart rate (RHR), and respiratory rate (RR) without intervention, providing invaluable insights along the way.
Having achieved FDA 510(k) Clearance, Xandar Kardian is granted marketing authorization for the XK300 Autonomous Health Monitoring Solution, showcasing heightened confidence in the product at a time where demand for radar technology is surging.
Simple to use and designed to work on its own, Xandar Kardian uses radar technology to detect micro vibrational frequency patterns being emitted from the body. The solution obtains a baseline RHR and RR by continuously tracking measurements over time, customizing a standard threshold based upon physician discretion and patient pre-conditions. Once the thresholds are engaged, the system can alert medical staff in emergent and non-emergent events via integrated nurse call systems, cloud-based mobile applications, or dashboard push alerts.
“Since 2011, our teams have been locked-in on perfecting digital radar signal processing solutions, providing a much-needed upgrade to current solutions in today’s fast-paced, tech-savvy world,” said Sam Yang, Managing Director of Xandar Kardian. “We are thrilled to share that after eight years of research and development, we have officially obtained FDA 510(k) Clearance, giving us the opportunity to share our robust, low-cost, revolutionary technology with the medical field, providing a groundbreaking technology that measures both resting heart rate and respiratory rates quickly and accurately.”
Xandar Kardian’s technology reduces both the physical and mental stress of conducting regular spot checks, with no staff intervention required. This form of non-contact monitoring means less chances of infection between patients and nurses, ideal in a post-COVID-19 era. Particularly useful for the treatment of chronic respiratory disorders, Xandar Kardian’s radar-based monitoring can provide valuable information on a patient’s respiratory and cardiovascular health. The XK300 qualifies for Medicare via CPT Code 99454, alleviating all out-of-pocket patient costs for those that qualify, while the prescribing physician receives an average of $69 per month.
Beyond healthcare applications, Xandar Kardian is tremendously beneficial in smart building implementation, occupancy regulation, and providing an upgrade to the standard PIR motion sensor systems by discerning human presence in any given room, effectively powering down as soon as RR becomes undetectable.
Xandar Kardian’s XK300 received significant validation at CES 2021 as a recipient of the CES Innovation Award, an annual competition honoring outstanding design and engineering in consumer technology products. The FDA 510(k) Clearance represents another milestone in bringing this essential technology to the mainstream.
To find more information on Xandar Kardian and the XK300 Autonomous Health Monitoring Solution, visit the Xandar Kardian website at www.kardian.com.