Scientists demonstrate that a bilateral tumor model could be useful to investigate the relationship between T-cell repertoire and the therapeutic effects of cancer immunotherapy
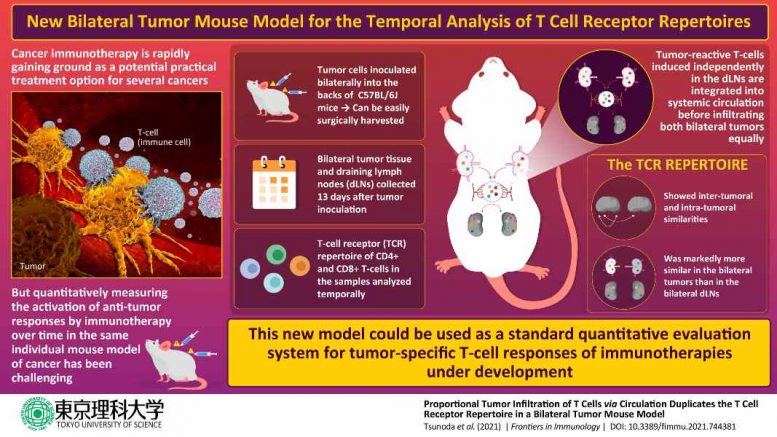
Using samples from 8-week-old mice with induced bilateral tumors on the left and right sides of their bodies, the scientists isolated the tumors and draining lymph nodes (dLNs) and examined their T-cell population and repertoire, using techniques such as cell sorting and “next-generation sequencing.” They were amazed to find that the T-cell profiles of both tumors (left and right) were almost identical, with strikingly similar T-cell clonal abundance (the proportions of different subtypes of T-cells) and repertoire, which indicate a similar anti-tumor response in a single mouse. “This proves that the T cell responses on one side reflects those on the other side in our bilateral tumor model”, Dr. Ueha mentions, motivated by the findings.
Also noteworthy is the fact that the T-cell repertoires of separate mice differed dramatically, and the variance between two tumors and within a single tumor was identical. Dr. Ueha and his colleagues speculated that the T-cells from the dLNs infiltrate both tumors after their distribution via blood circulation. “This was important to investigate since the anticancer immune responses in clinical studies are studied longitudinally using biopsies from the same tumor, but our model uses two different tumors”, Prof. Matsushima explains.
To test their hypothesis, the team examined the T-cell population in the two dLNs and their corresponding tumors, each on the left and right side, and found that the overlaps between dLNs and the tumor were seemingly high, but that between two dLNs was poor. “In clinical practice, cancers often invade or metastasize to multiple sites, and our results suggest that even independent tumors may have similar immune responses occurring at the tumor site,” Dr. Ueha suggests.
When asked about their future research plans, Dr. Ueha says, “We have plans to combine the single-cell TCR repertoire analysis and bilateral tumor model to understand the fate and immunological significance of T-cells with various tumor-reactivity in response to cancer immunotherapy. Our model would be applicable to other tumor models since the conserved TCR repertoire appears to be based on a mechanism that is conserved across individuals and species.
The group assumes that TCRs, like unique barcodes, can be read by high-throughput sequencing to develop “biomarkers” for tumor-specific immune responses, and optimize ICI-based immunotherapy. “The TCR repertoire analysis using our bilateral mouse model is expected to contribute to the development of new cancer immunotherapies for quantitative analysis of tumor-specific T cell responses,” Dr. Ueha concludes.