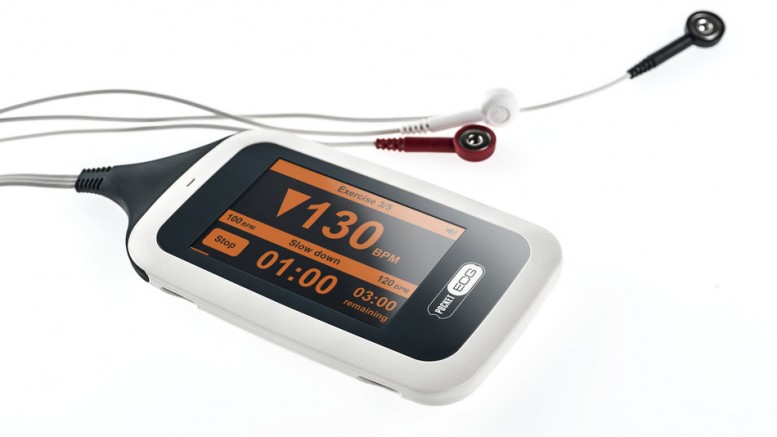
New Device offers Data Critical to Effective Rehab Training
MEDICALgorithmics, S.A. (WSE: MDG) and U.S. subsidiary Medi-Lynx Cardiac Monitoring L.L.C., today announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the PocketECG Cardiac Rehabilitation System (CRS), a new mobile cardiac rehabilitation system designed to provide high-quality ECG monitoring and automated arrhythmia detection during rehabilitation training. The device is currently approved and marketed in the European Union and patented in the US (US Patent No. 9,846,764) for use in low and high-risk cardiac patients.
“We are thrilled to now introduce PocketECG CRS to patients and clinicians in the U.S., providing a valuable monitoring tool with the potential to improve the effectiveness of cardiac rehabilitation training,” said Marek Dziubinski, PhD, CEO of MEDICALgorithmics. “PocketECG CRS was built on our PocketECG arrhythmia monitoring solution platform, and includes new software and enhancements specifically designed for use during all phases of the cardiac rehabilitation process – from early mobilization during hospitalization to post-discharge and ongoing maintenance.”
PocketECG CRS is a smartphone-sized device that monitors a patient’s heart rhythm and heart rate during rehabilitation exercises to safely guide the intensity and duration of workouts in real-time. The system transmits the full disclosure ECG signal classifying every heartbeat to automatically detect abnormalities and arrhythmia. A built-in accelerometer allows clinicians to analyze the impact of physical activity on heart rate. All data are streamed via mobile telephony network to a secure online clinician portal and to a cardiac monitoring center for constant monitoring, evaluation, and urgent notifications.
CR or medically supervised exercise training is highly recommended by the American Heart Association and shown to improve the health and recovery of patients with acute myocardial infarction or heart attack, chronic stable angina, coronary artery bypass grafting (CABG), percutaneous coronary intervention (PCI), cardiac valve surgery, stable, chronic heart failure, and cardiac transplantation. The American Association of Cardiovascular and Pulmonary Rehabilitation estimates that at least 3.8 million patients are eligible to receive CR.