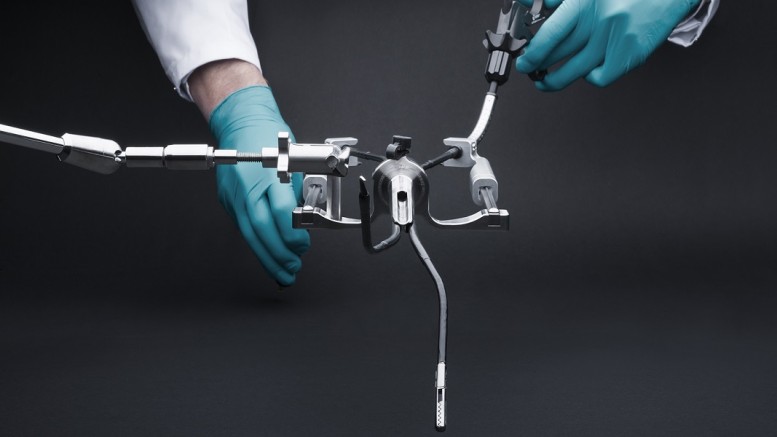
Following successful use of FMX314 in general surgery, the innovative platform for single-port laparoscopy has now been employed in several gynecologic procedures including tubal sterilization and ovarian cyst treatment with positive results, confirming the versatility of the platform for other surgical applications.
FMX314 is the world’s first single-port surgery solution that is compatible with a standard 15mm laparoscopic trocar, promising fewer port-site complications, less post-operative pain, faster recovery and exceptional cosmesis. A procedure with the platform closely mimics conventional, multi-port laparoscopy, which makes FMX314 easy to use.
Market introduction of FMX314 in the United States for abdominal laparoscopic surgery is expected in the fourth quarter of this year, followed by commercial launch in Europe.
Caution: The FMX314 Surgical Platform is an investigational device and not available for sale.
About Fortimedix Surgical
Fortimedix Surgical is a fast growing medical device product definition company, aiming to challenge the status quo in minimally invasive surgery by creating novel devices that capture the claimed benefits of single-port surgery. The company is headquartered in Nuth, The Netherlands, and has a US subsidiary in San Diego, CA. With a strong history as a global market leader in contract stent manufacturing, Fortimedix has been a trusted partner in the medical device industry for over 15 years. Fortimedix Surgical is committed to continuing the evolution of minimally invasive surgery by driving technological innovation across surgical specialties to improve the quality of life of patients throughout the world.
For more information, please visit www.fortimedixsurgical.com