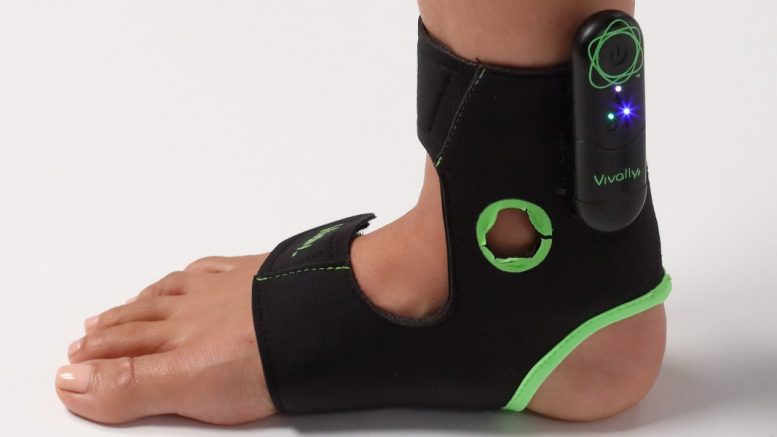
Avation’s Vivally System, the only FDA-cleared, non-invasive, closed-loop, at-home wearable, changes the way urinary incontinence and urgency caused by overactive bladder syndrome is treated
Avation Medical, a company focused on neuromodulation technologies and digital health has closed more than $22 million in equity financing in its oversubscribed Series C round, led by ShangBay Capital and Asahi Kasei, and included Angelini Ventures, JobsOhio Growth Capital Fund, among others. Existing investors, including Arboretum Ventures, Tonkawa, Medtronic and Avestria Ventures also participated in the latest round. The funding will be used for the U.S. launch of the Vivally System.
The Vivally System is the only non-invasive, FDA-cleared, wearable neuromodulation system for home use that delivers closed-loop, autonomously adjusted electrical stimulation to treat patients with urge urinary incontinence (UUI) and urinary urgency caused by overactive bladder (OAB) syndrome, which affects more than 46 million people in the U.S. Using proprietary algorithms and electromyographical sensors, the System detects and calibrates to the level of energy being delivered to the tibial nerve during stimulation to ensure optimal, customized therapeutic output in a true real-time, closed-loop operation. Worn on the ankle, Vivally is used at home for therapy sessions lasting only 30 minutes, as little as once per week.
Unlike other implantable neuromodulation approaches for bladder treatment, Vivally requires no surgery and no drugs. Avation Co-Founder and CEO Jill Schiaparelli confirms, “This new capital will jump-start the highly anticipated launch of Vivally, which sets a new standard for OAB care. We are thrilled to welcome new investors and grateful for the unwavering support of existing investors who share our belief that non-invasive wearable neuromodulation therapy will significantly benefit patients and clinicians. We could not ask for a stronger group of institutional investors as we accelerate our efforts to bring this innovative technology to market.” Schiaparelli noted that representatives from Asahi Kasei and Angelini Ventures will now join Avation Medical’s board of directors.
A surgery- and drug-free at-home wearable neuromodulation system represents a huge advancement in the treatment of OAB. While neuromodulation has traditionally been effective in treating OAB symptoms, to date it has required in-office visits or invasive surgical procedures, relegating it to less than 10 percent of patients seeking treatment. Vivally can be used earlier in the care pathway to treat the approximately nine million people seeking treatment annually for their symptoms. In two multi-center clinical trials, the Vivally System was shown to significantly reduce daily void, incontinence, and urgency episodes and improve patient quality of life. Additionally, the System facilitated an 88-percent therapy compliance rate. Symptom reduction was demonstrated out to one year, even with a decrease in therapy frequency. Full details of the study were recently published in the journal Urology.
Avation Medical is an innovative neuromodulation and digital health company with a mission to make wearable peripheral neuromodulation accessible to patients across a variety of clinical conditions.